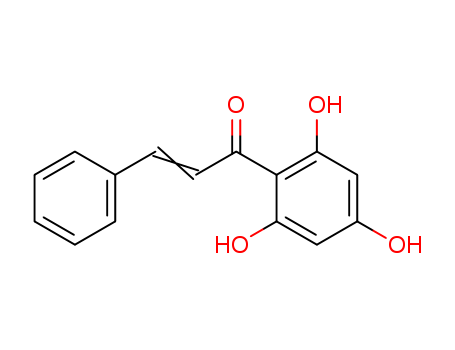
- Chemical Name:Pinocembrin chalcone
- CAS No.:4197-97-1
- Molecular Formula:C15H12O4
- Molecular Weight:256.258
- Hs Code.:
- DSSTox Substance ID:DTXSID101343555
- Nikkaji Number:J258.619C
- Wikidata:Q27149535
- Metabolomics Workbench ID:26719
- ChEMBL ID:CHEMBL129371
- Mol file:4197-97-1.mol
Synonyms:2',4',6'-trihydroxychalcone;pinocembrin chalcone