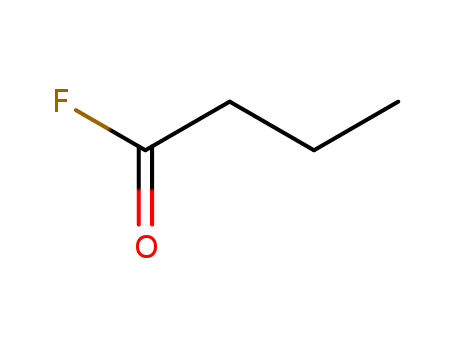
- Chemical Name:Butanoyl fluoride
- CAS No.:461-53-0
- Molecular Formula:C4H7 F O
- Molecular Weight:90.0974
- Hs Code.:2915900090
- European Community (EC) Number:207-310-7
- DSSTox Substance ID:DTXSID2060040
- Nikkaji Number:J196.178K
- Wikidata:Q81989041
- Mol file:461-53-0.mol
Synonyms:Butanoyl fluoride;Butyryl fluoride;461-53-0;EINECS 207-310-7;C4H7FO;DTXSID2060040


 Xi,
Xi, C
C


