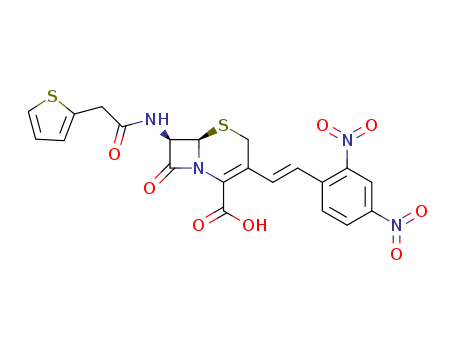
- Chemical Name:Nitrocefin
- CAS No.:41906-86-9
- Molecular Formula:C21H16N4O8S2
- Molecular Weight:516.512
- Hs Code.:29349990
- European Community (EC) Number:636-988-0
- UNII:EWP54G0J8F
- DSSTox Substance ID:DTXSID401318525
- Nikkaji Number:J17.395I
- Wikipedia:Nitrocefin
- Wikidata:Q1993962
- NCI Thesaurus Code:C76562
- Metabolomics Workbench ID:152736
- ChEMBL ID:CHEMBL480517
- Mol file:41906-86-9.mol
Synonyms:Cefinase;glaxo 87-312;nitrocefin;nitrocefin, (6R-trans)-isomer;nitrocefin, monosodium salt;nitrocefin, sodium salt, (6R-trans)-isomer