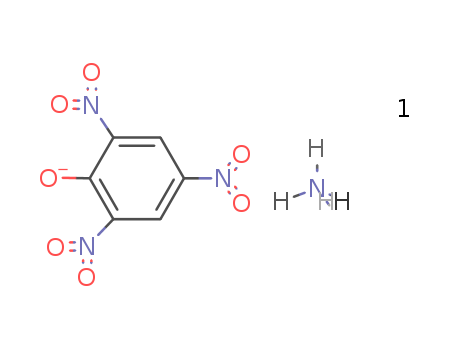
- Chemical Name:Ammonium 2,4,6-trinitrophenolate
- CAS No.:131-74-8
- Molecular Formula:C6H3 N3 O7 . H3 N
- Molecular Weight:246.136
- Hs Code.:2923900090
- Wikidata:Q424516
- Mol file:131-74-8.mol
Synonyms:ammonium 2,4,6-trinitrophenolate;azane;2,4,6-trinitrophenol;picric acid, ammonia salt