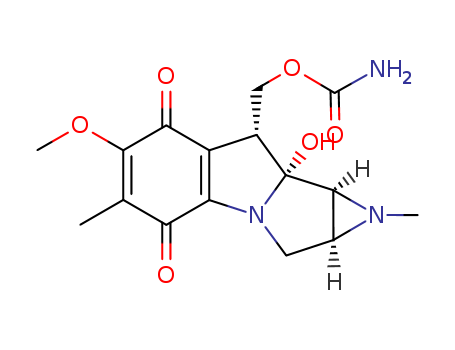
- Chemical Name:Mitomycin B
- CAS No.:4055-40-7
- Molecular Formula:C16H19 N3 O6
- Molecular Weight:349.343
- Hs Code.:
- UNII:VT1IGL3D7V
- DSSTox Substance ID:DTXSID001318185
- Nikkaji Number:J55.724B
- Wikidata:Q22252155
- NCI Thesaurus Code:C1392
- Metabolomics Workbench ID:102726
- ChEMBL ID:CHEMBL323536
- Mol file:4055-40-7.mol
Synonyms:mitomycin A;mitomycin A, (1aS-(1aalpha,8alpha,8aalpha,8balpha))-isomer;mitomycin B