Base Information
Edit
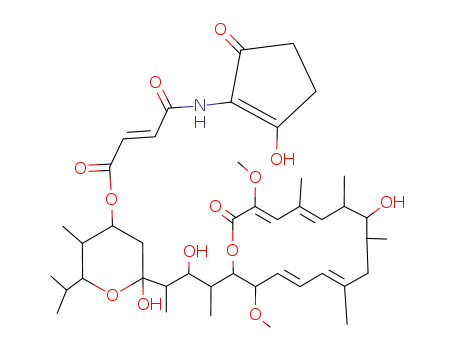
- Chemical Name:BAFILOMYCIN B1
- CAS No.:88899-56-3
- Molecular Formula:C44H65NO13
- Molecular Weight:815.999
- Hs Code.:2941900000
- European Community (EC) Number:635-481-1
- Wikidata:Q105140567
- Mol file:88899-56-3.mol
Synonyms:Hygrolidin,37-decarboxy-2-demethyl-37-[[(2-hydroxy-5-oxo-1-cyclopenten-1-yl)amino]carbonyl]-2-methoxy-24-methyl-;Oxacyclohexadecane, hygrolidin deriv.; 2-Butenoic acid,4-[(2-hydroxy-5-oxo-1-cyclopenten-1-yl)amino]-4-oxo-,tetrahydro-2-hydroxy-2-[2-hydroxy-3-(10-hydroxy-3,15-dimethoxy-7,9,11,13-tetramethyl-16-oxooxacyclohexadeca-4,6,12,14-tetraen-2-yl)-1-methylbutyl]-5-methyl-6-(1-methylethyl)-2H-pyran-4-ylester,[2R-[2R*[1S*[2R*,4R*(E),5S*,6R*],2R*,3S*],3S*,4E,6E,9S*,10S*,11R*,12E,14Z]]-;Bafilomycin B1; Setamycin



 T+
T+
