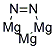Base Information
Edit
- Chemical Name:MAGNESIUM NITRIDE
- CAS No.:12057-71-5
- Molecular Formula:Mg3N2
- Molecular Weight:100.93
- Hs Code.:
- Mol file:12057-71-5.mol

Synonyms:Magnesiumnitride; Trimagnesium dinitride