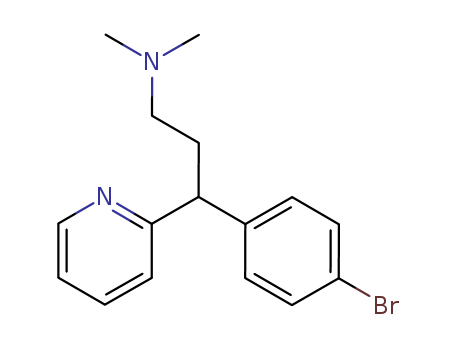
- Chemical Name:Dexbrompheniramine
- CAS No.:132-21-8
- Molecular Formula:C16H19BrN2
- Molecular Weight:319.244
- Hs Code.:
- European Community (EC) Number:205-053-5
- UNII:75T64B71RP
- DSSTox Substance ID:DTXSID8022905
- Wikipedia:Dexbrompheniramine
- Wikidata:Q5268318
- NCI Thesaurus Code:C61705
- RXCUI:22696
- Pharos Ligand ID:24AH7CACD7SV
- Metabolomics Workbench ID:42766
- ChEMBL ID:CHEMBL1201287
- Mol file:132-21-8.mol
Synonyms:dexbrompheniramine;dexbrompheniramine maleate;Disophrol