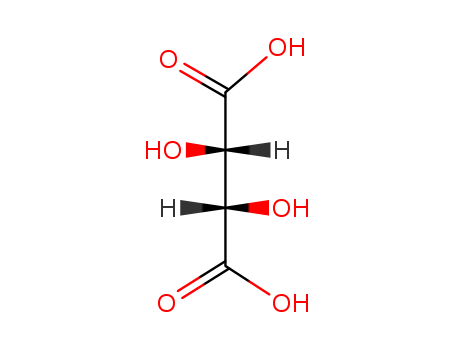
- Chemical Name:d-Tartaric acid
- CAS No.:147-71-7
- Deprecated CAS:1150316-16-7
- Molecular Formula:C4H6O6
- Molecular Weight:150.088
- Hs Code.:29181200
- European Community (EC) Number:205-695-6
- ICSC Number:0772
- UNII:RRX6A4PL3C
- DSSTox Substance ID:DTXSID4043775,DTXSID5046986
- Nikkaji Number:J9.264I
- Wikipedia:Tartaric acid,Tartaric_acid,Tartrate
- Wikidata:Q23034947
- NCI Thesaurus Code:C47744
- RXCUI:37578
- Metabolomics Workbench ID:44098
- ChEMBL ID:CHEMBL1200861
- Mol file:147-71-7.mol
Synonyms:(R*,R*)-(+-)-2,3-dihydroxybutanedioic acid, monoammonium monosodium salt;aluminum tartrate;ammonium tartrate;calcium tartrate;calcium tartrate tetrahydrate;Mn(III) tartrate;potassium tartrate;seignette salt;sodium ammonium tartrate;sodium potassium tartrate;sodium tartrate;stannous tartrate;tartaric acid;tartaric acid, ((R*,R*)-(+-))-isomer;tartaric acid, (R*,S*)-isomer;tartaric acid, (R-(R*,R*))-isomer;tartaric acid, (S-(R*,R*))-isomer;tartaric acid, ammonium sodium salt, (1:1:1) salt, (R*,R*)-(+-)-isomer;tartaric acid, calcium salt, (R-R*,R*)-isomer;tartaric acid, monoammonium salt, (R-(R*,R*))-isomer;tartrate


 Xi
Xi


