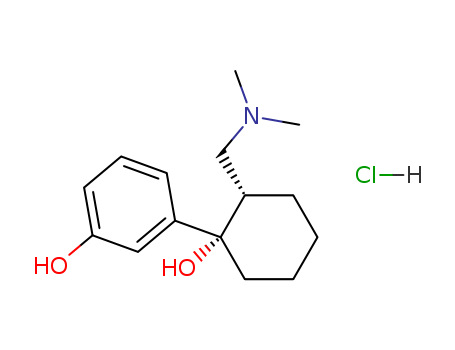
- Chemical Name:O-Desmethyl Tramadol Hydrochloride
- CAS No.:16412-54-7
- Molecular Formula:C15H23NO2.ClH
- Molecular Weight:285.814
- Hs Code.:2922509090
- UNII:XL5VDC061E
- Wikidata:Q27293887
- Mol file:16412-54-7.mol
Synonyms:O-demethyl tramadol;O-demethyltramadol;O-demethyltramadol hydrochloride;O-desmethyltramadol;tramadol M1 metabolite