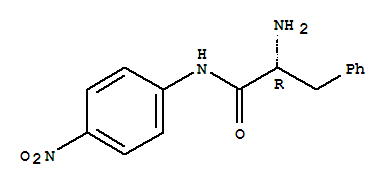
- Chemical Name:H-D-Phe-Pna
- CAS No.:14235-18-8
- Molecular Formula:C15H15 N3 O3
- Molecular Weight:285.302
- Hs Code.:2924299090
- DSSTox Substance ID:DTXSID70428590
- Wikidata:Q82241415
- Mol file:14235-18-8.mol
Synonyms:H-D-Phe-Pna;14235-18-8;(2R)-2-amino-N-(4-nitrophenyl)-3-phenylpropanamide;D-Phenylalanine 4-nitroanilide;DTXSID70428590;MFCD00077153;N-(4-Nitrophenyl)-D-phenylalaninamide;AS-46825;F10634;A885191