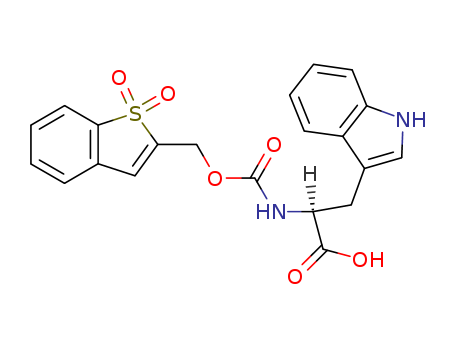
- Chemical Name:N-Bsmoc-L-tryptophan
- CAS No.:197245-27-5
- Molecular Formula:C21H18N2O6S
- Molecular Weight:426.45
- Hs Code.:
- DSSTox Substance ID:DTXSID10428832
- Nikkaji Number:J1.135.234K
- Wikidata:Q82241664
- Mol file:197245-27-5.mol
Synonyms:N-Bsmoc-L-tryptophan;197245-27-5;(2S)-2-[(1,1-dioxo-1-benzothiophen-2-yl)methoxycarbonylamino]-3-(1H-indol-3-yl)propanoic acid;DTXSID10428832;AKOS024348919