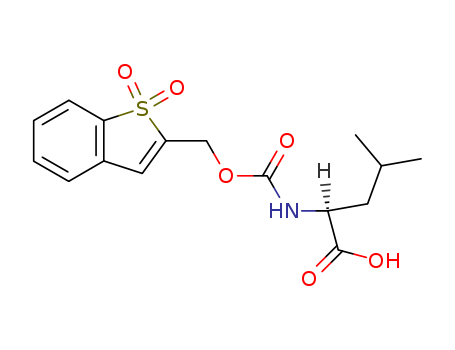
- Chemical Name:N-Bsmoc-L-leucine
- CAS No.:197245-21-9
- Molecular Formula:C16H19NO6S
- Molecular Weight:353.396
- Hs Code.:
- DSSTox Substance ID:DTXSID80428829
- Nikkaji Number:J1.135.228F
- Wikidata:Q82241660
- Mol file:197245-21-9.mol
Synonyms:N-Bsmoc-L-leucine;197245-21-9;(2S)-2-[(1,1-dioxo-1-benzothiophen-2-yl)methoxycarbonylamino]-4-methylpentanoic acid;(((1,1-Dioxidobenzo[b]thiophen-2-yl)methoxy)carbonyl)-L-leucine;starbld0001494;DTXSID80428829;AKOS024348910;N-[(1-Benzothiophene 1,1-dioxide)-2-ylmethoxycarbonyl]-L-leucine;N-(Benzo[b]thiophenesulfone-2-methoxycarbonyl)-L-leucine Bsmoc-Leu-OH