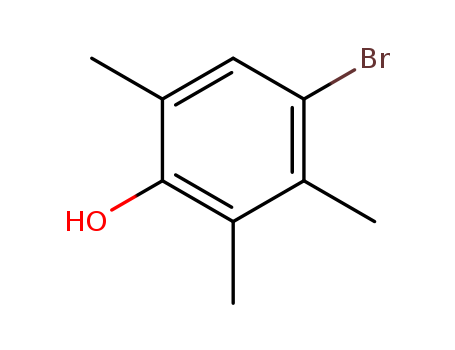
- Chemical Name:4-Bromo-2,3,6-trimethylphenol
- CAS No.:51857-41-1
- Molecular Formula:C9H11BrO
- Molecular Weight:215.09
- Hs Code.:2908199090
- DSSTox Substance ID:DTXSID30445384
- Wikidata:Q82263760
- Mol file:51857-41-1.mol
Synonyms:4-bromo-2,3,6-trimethylphenol;51857-41-1;4-bromo-2,3,6-trimethyl-phenol;SCHEMBL258837;DTXSID30445384;CNZUMQRUUPCHJR-UHFFFAOYSA-N;4-bromo-2,3,6-trimethyl phenol;4-AMINO-3-FORMYLPYRIDINEHCL;BCA85741;MFCD00983556;AKOS015965943;CS-W004396;AC-20928;BS-16954;D84235