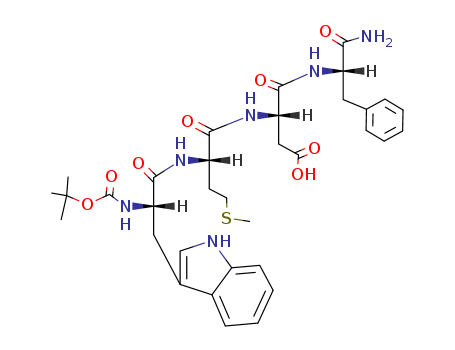
- Chemical Name:Boc-trp-met-asp-phe-NH2
- CAS No.:5235-21-2
- Molecular Formula:C34H44 N6 O8 S
- Molecular Weight:696.825
- Hs Code.:
- European Community (EC) Number:226-032-7
- ChEMBL ID:CHEMBL64605
- Nikkaji Number:J297.948I
- Mol file:5235-21-2.mol
Synonyms:Boc-CCK-4;Boc-CCK4;Boc-Trp-Met-Asp-Phe-NH2;butyloxycarbonyl-tryptophyl-methionyl-aspartyl-phenylalaninamide