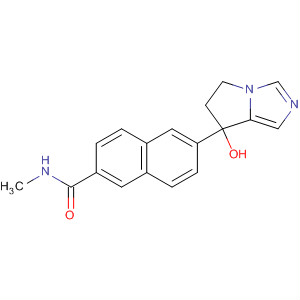
- Chemical Name:Orteronel
- CAS No.:426219-18-3
- Molecular Formula:C18H17N3O2
- Molecular Weight:307.34600
- Hs Code.:
- European Community (EC) Number:687-683-4
- UNII:UE5K2FNS92
- DSSTox Substance ID:DTXSID001319121
- Nikkaji Number:J2.839.701A
- Wikipedia:Orteronel
- Wikidata:Q76411446
- NCI Thesaurus Code:C90582
- Pharos Ligand ID:BX733GLTC3XD
- Metabolomics Workbench ID:153122
- ChEMBL ID:CHEMBL1921976
- Mol file:426219-18-3.mol
Synonyms:6-((7S)-7-hydroxy-6,7-dihydro-5H-pyrrolo(1,2-c)imidazol-7-yl)-N-methyl-2-naphthamide;orteronel;TAK-700