Base Information
Edit
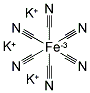
- Chemical Name:Potassium ferricyanide
- CAS No.:13746-66-2
- Deprecated CAS:2002-18-8,409-16-5,1419873-89-4
- Molecular Formula:K3Fe.(CN)6
- Molecular Weight:329.27
- Hs Code.:28372000
- European Community (EC) Number:237-323-3
- ICSC Number:1132
- Wikipedia:Potassium ferricyanide,Potassium_ferricyanide
- Mol file:13746-66-2.mol

Synonyms:K3Fe(CN)6;potassium ferricyanide;potassium hexacyanoferrate (III)


 Xn
Xn
