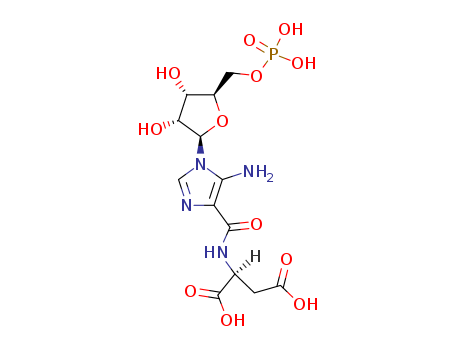
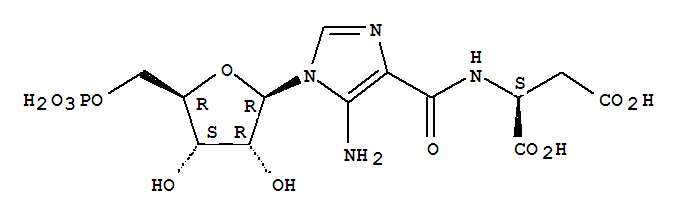
- Chemical Name:Saicar
- CAS No.:3031-95-6
- Molecular Formula:C13H19 N4 O12 P
- Molecular Weight:454.287
- Hs Code.:
- UNII:K1PVR64RIF
- DSSTox Substance ID:DTXSID90184404
- Nikkaji Number:J7.666J
- Wikipedia:Phosphoribosylaminoimidazolesuccinocarboxamide
- Wikidata:Q3502778
- Metabolomics Workbench ID:37432
- Mol file:3031-95-6.mol
Synonyms:5'-phosphoribosyl-4-(N-succinylcarboxamide)-5-aminoimidazole;5-amino-4-imidazole-N-succinocarboxamide ribonucleotide;N-(5-amino-1-beta-D-ribofuranosylimidazole-4-carbonyl)-L-aspartic acid 5'-phosphate;SAICAR;SAICAR, (D)-isomer;succinyl-AICAR