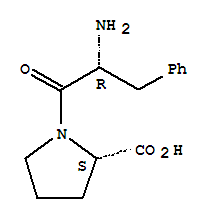
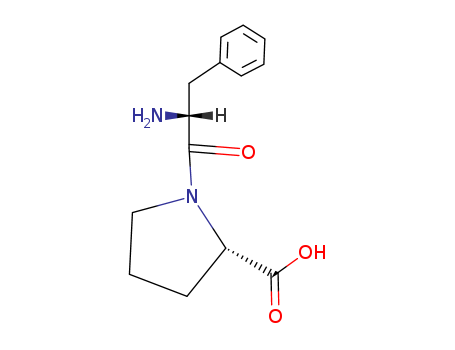
- Chemical Name:H-D-PHE-PRO-OH TRIFLUOROACETATE SALT
- CAS No.:51926-52-4
- Molecular Formula:C14H18 N2 O3
- Molecular Weight:262.309
- Hs Code.:2933990090
- Mol file:51926-52-4.mol
Synonyms:L-Proline,1-D-phenylalanyl-; 147: PN: WO2005000193 SEQID: 147 claimed sequence; 32: PN:EP2085120 SEQID: 32 claimed sequence; 32: PN: WO2009095265 SEQID: 32 claimedsequence; D-Phenylalanyl-L-proline