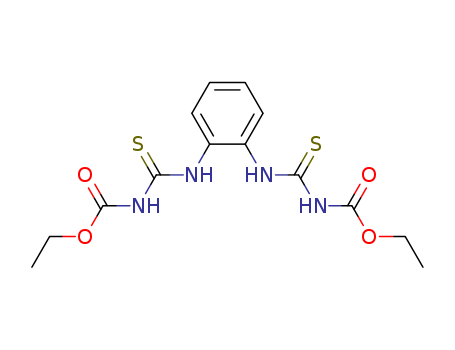
- Chemical Name:Thiophanate
- CAS No.:23564-06-9
- Deprecated CAS:39300-54-4,37233-54-8,37359-51-6,37359-51-6
- Molecular Formula:C14H18 N4 O4 S2
- Molecular Weight:370.453
- Hs Code.:29309090
- European Community (EC) Number:245-741-2
- NSC Number:170810
- UN Number:2588
- UNII:5Q0Y96D5I8
- DSSTox Substance ID:DTXSID3034531
- Nikkaji Number:J20.160J
- Wikidata:Q27155716
- NCI Thesaurus Code:C76880
- ChEMBL ID:CHEMBL2103777
- Mol file:23564-06-9.mol
Synonyms:Bis-Thioallophanate, Dimethylphenylene;Cercobin M 70;Cercobin M-70;Cercobin M70;Dimethylphenylene Bis Thioallophanate;Dimethylphenylene Bis-Thioallophanate;Mildothane;Thiophanate;Thiophanate Methyl;Thiophanate-Methyl