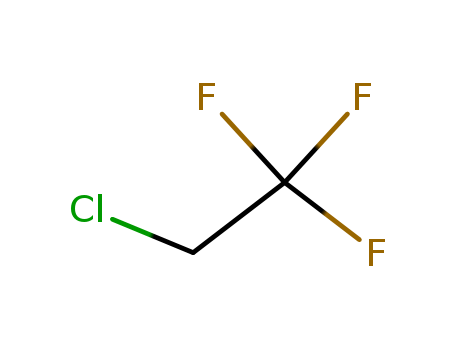
- Chemical Name:2-Chloro-1,1,1-trifluoroethane
- CAS No.:75-88-7
- Molecular Formula:C2H2 Cl F3
- Molecular Weight:118.486
- Hs Code.:2903791013
- European Community (EC) Number:200-912-0
- ICSC Number:1299
- UN Number:1983
- UNII:H86O899T9B
- DSSTox Substance ID:DTXSID5020289
- Nikkaji Number:J83.985J
- Wikipedia:2-Chloro-1,1,1-trifluoroethane
- Wikidata:Q3596740,Q83053679
- ChEMBL ID:CHEMBL142556
- Mol file:75-88-7.mol
Synonyms:1,1,1-trifluoro-2-chloroethane;1-chloro-2,2,2-trifluoroethane;2-chloro-1,1,1,-trifluoroethane;2-CTE;FC-133a



 Xi
Xi

