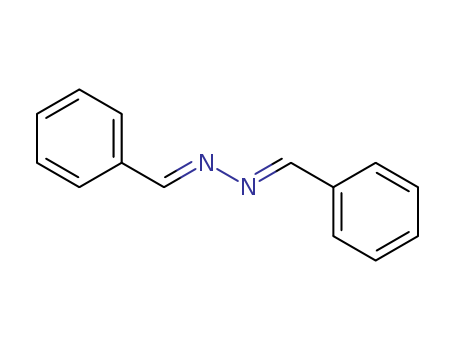
- Chemical Name:Benzalazine
- CAS No.:28867-76-7
- Molecular Formula:C14H12 N2
- Molecular Weight:208.263
- Hs Code.:2928000090
- European Community (EC) Number:209-627-6,631-102-9
- NSC Number:3269
- UNII:G68LHD5XNY
- ChEMBL ID:CHEMBL3199194
- Mol file:28867-76-7.mol
Synonyms:benzalazine