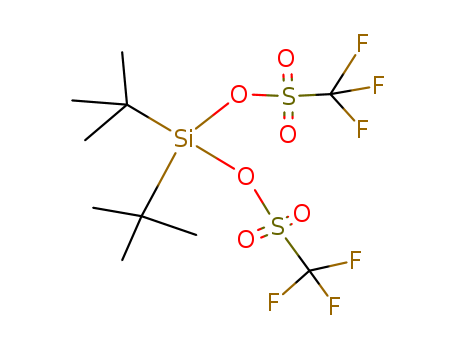
- Chemical Name:Di-tert-butylbis(trifluoromethanesulfonyloxy)silane
- CAS No.:85272-31-7
- Molecular Formula:C10H18F6O6S2Si
- Molecular Weight:440.457
- Hs Code.:29319090
- European Community (EC) Number:680-172-7
- DSSTox Substance ID:DTXSID50337994
- Nikkaji Number:J364.545B
- Wikidata:Q72470111
- Mol file:85272-31-7.mol
Synonyms:85272-31-7;di-tert-Butylsilyl bis(trifluoromethanesulfonate);Di-tert-butylbis(trifluoromethanesulfonyloxy)silane;Di-tert-butylsilanediyl bis(trifluoromethanesulfonate);[ditert-butyl(trifluoromethylsulfonyloxy)silyl] trifluoromethanesulfonate;DTBS ditriflate;Di-tert-butylsilyl ditriflate;Di-tert-butylsilylBis(trifluoromethanesulfonate);Di-t-butylsilybis(trifluoromethanesulfonate;Bis(trifluoromethanesulfonic acid)di-tert-butylsilanediyl ester;di-t-butylsilylbis(trifluoromethanesulfonate);di-t-butylsilyl bis(trifluoromethanesulfonate);Trifluoromethanesulfonic acid di-tert-butylsilylene ester;C10H18F6O6S2Si;SCHEMBL12936595;DTXSID50337994;MFCD00010581;AKOS015837779;CS-W009662;FS-4632;AC-32462;D3135;FT-0625367;D78536;S05900;Di-tert-butylbis(trifluoromethylsulfonyloxy)silane;A841277;di-tert-butylsilyl bis(trifluoromethane sulfonate);di-tert-butylsilyl bis(trifluoromethanesulphonate);Bis(tert-butyl)silyl bis(trifluoromethanesulphonate);Di-tert-butylsilyl bis(trifluoromethanesulfonate), 97%;[Di-tert-butyl(trifluoromethylsulfonyloxy)silyl] trifluoromethanesulfonate;Di-tert-butylsilyl bis(trifluoromethanesulfonate), purum, >=96.0% (GC);Methanesulfonic acid, trifluoro-, bis(1,1-dimethylethyl)silylene ester;Di(tert-butyl)([(trifluoromethyl)sulfonyl]oxy)silyl trifluoromethanesulfonate #;Di-tert-butylsilyl ditriflate DTBS ditriflate Trifluoromethanesulfonic acid di-tert-butylsilylene ester