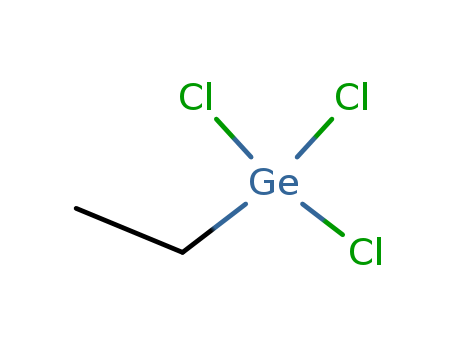
- Chemical Name:Ethylgermanium trichloride
- CAS No.:993-42-0
- Molecular Formula:C2H5Cl3Ge
- Molecular Weight:208.011
- Hs Code.:
- European Community (EC) Number:633-711-5
- DSSTox Substance ID:DTXSID30369859
- Nikkaji Number:J1.118.653J
- Wikidata:Q41751073
- Mol file:993-42-0.mol
Synonyms:Ethylgermanium trichloride;993-42-0;trichloro(ethyl)germane;ETHYLTRICHLOROGERMANE;MFCD00013586;SCHEMBL302154;DTXSID30369859;AKOS015915247;FT-0742336



 C
C


