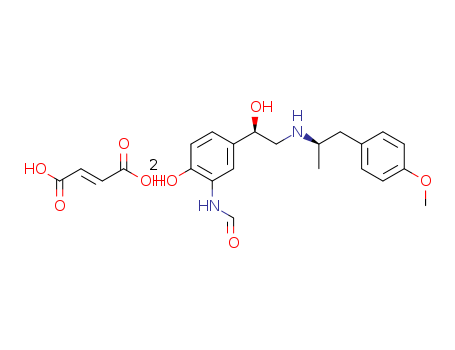
- Chemical Name:Formoterol hemifumarate
- CAS No.:87833-61-2
- Deprecated CAS:83536-10-1,87481-49-0,87833-61-2,87481-49-0,87833-61-2
- Molecular Formula:C4H4O4*2C19H24N2O4
- Molecular Weight:804.894
- Hs Code.:
- European Community (EC) Number:610-119-5
- UNII:P3T5QA5J9N
- DSSTox Substance ID:DTXSID5044239
- Wikidata:Q27132391
- NCI Thesaurus Code:C47540
- RXCUI:236216
- ChEMBL ID:CHEMBL3184383
- Mol file:87833-61-2.mol
Synonyms:3-formylamino-4-hydroxy-alpha-(N-1-methyl-2-p-methoxyphenethylaminomethyl)benzyl alcohol.hemifumarate;arformoterol;BD 40A;eformoterol;Foradil;formoterol;formoterol fumarate;formoterol fumarate, ((R*,R*)-(+-))-isomer;formoterol, ((R*,R*)-(+-))-isomer;Oxis